

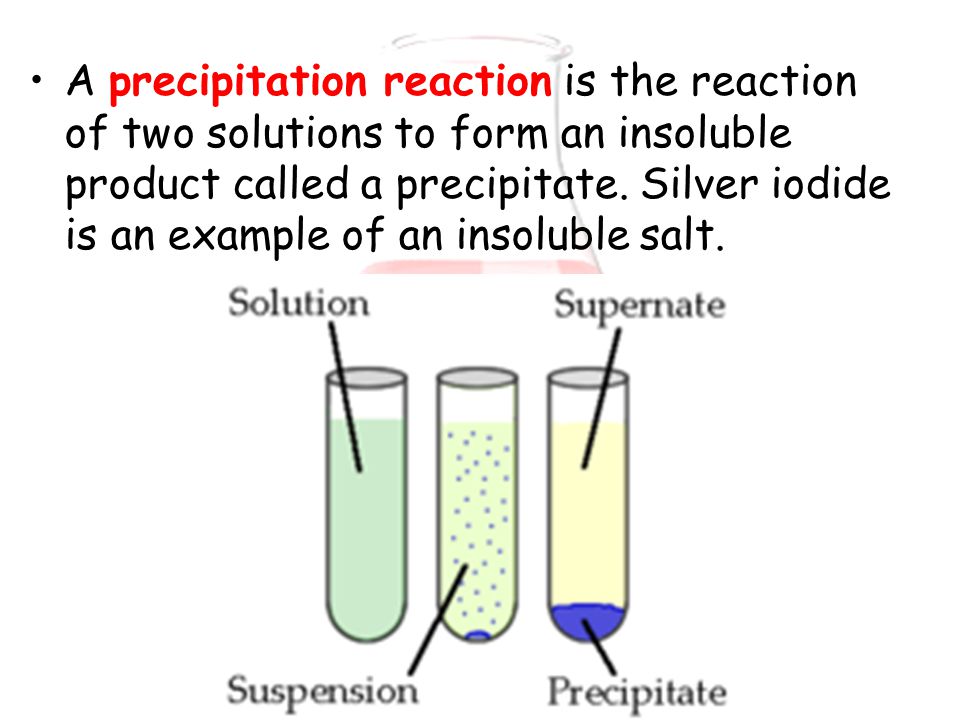
Since Na +(aq) and NO 3 - do not participate in the reaction they are called spectator ions. If more sodium hydroxide solution is added, the precipitate redissolves to give a. In the example above Na +(aq) and NO 3 - are present as both products and reactants. solution - and that will be assumed in all the following examples. Net ionic equations are found by writing the full equation and then eliminating the spectator ions spectator ions are ions that do not participate in the reaction. A "complete ionic " equation could be used:.A " molecular " equation could be used:.
The reaction can be described in a number of ways Since Ag + is now in solutionwith Cl - the two will combine to form AgCl, and the AgClwill precipitate from solution. The resulting solution contains Na +,Ag +, Cl -, and NO 3 -, butAgCl is not soluble in water. Definition of PRECIPITATE (verb): make something suddenly happen or start existing solid substance: become separate from liquid fall or be thrown. If two solutions are mixed together it is possible that two ionscould combine to form an insoluble ionic complex.Ī solution of silver nitrate is combined with a solution of sodiumchloride. Most sulfide (S -2), carbonate (CO 3 2-), chromate (CrO 4 2-), and phosphate (PO 4 -3) are only slightly soluble.The compounds Ba(OH) 2, Sr(OH) 2, and Ca(OH) 2 are marginally soluble. The important soluble hydroxides are NaOH and KOH. Most hydroxide salts are only slightly soluble.Notable exceptions are BaSO 4, PbSO 4, HgSO 4, and CaSO 4. Notable exceptions are salts containing the ions Ag +, Pb 2+, and Hg 2 2+. Most chloride, bromide and iodide salts are soluble.The chemical equation for this precipitation reaction is provided below. This is the insoluble salt formed as a product of the precipitation reaction. Most salts containing the alkali metal ions (Li +, Na +, K +, Cs +, Rb +) and the ammonium ion (NH 4 +) are soluble. One of the best examples of precipitation reactions is the chemical reaction between potassium chloride and silver nitrate, in which solid silver chloride is precipitated out.Most nitrate (NO 3 -) salts are soluble.Simple Rules for the Solubility of Salts in Water We will discuss enthalpy and bond energylater this semester). We will discuss entropy and latticeenergy second semester. Theinteraction between Pb 2+ and SO 4 2-is simply stronger than the attraction of the water to either thePb 2+ or SO 4 2- ( Bothenthalpy and entropy play a roll. If PbSO 4is added to water, none of the lead(II)sulfate will dissolve. For example, sometimes the pipes in our homes get clogged because precipitates of magnesium and calcium oxides have. Not all ionic compounds are soluble in water. Precipitation reaction occur all around us.


 0 kommentar(er)
0 kommentar(er)
